Remote patient monitoring company Withings Health Solutions, the professional healthcare division of Withings, announced that its BPM Pro 2 cellular blood pressure monitor has been cleared by the FDA.
The BPM Pro 2, which Withings released in October, is designed to measure blood pressure and pulse rate in adults with arm circumferences of 9 to 17 inches (22 cm to 42 cm) or 16 to 20 inches (40 to 52 cm).
It also permits care teams to scale remote patient monitoring and streamline operations by addressing a critical challenge in remote care, such as ensuring patients take more reliable at-home blood pressure measurements under prime conditions and supplying them with outcomes reported by the patient.
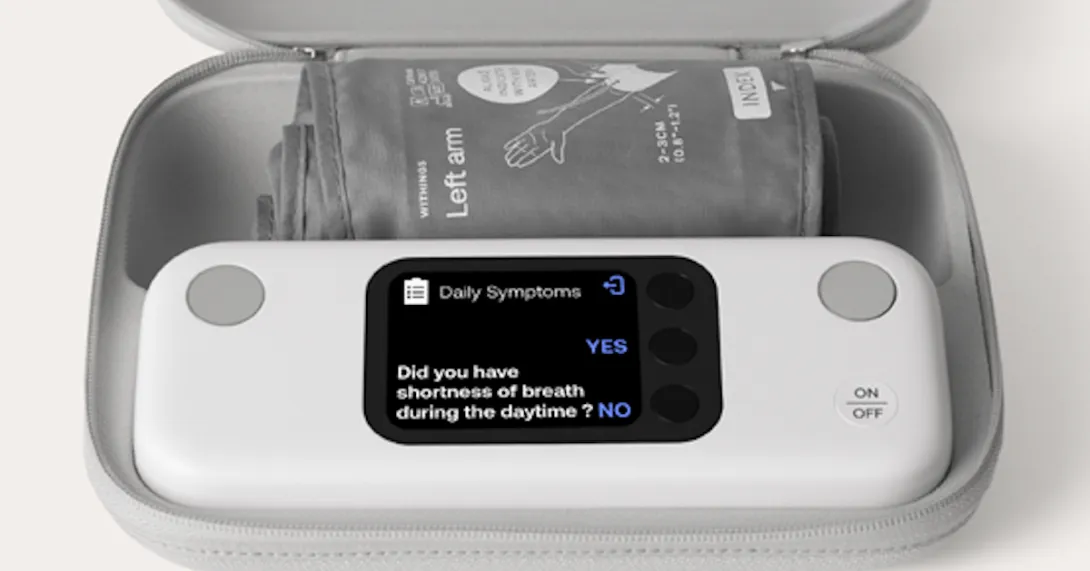
It includes a color screen that regularly educates patients on how to take the measurements properly, while adding a new level of insight and precision.
Questions can be asked to patients on the device's screen to capture information about symptoms and medication adherence through the Patient Insights feature.
Additionally, the Retake Measure feature reminds patients to retake a BP reading if the results exceed predetermined thresholds, which the company says can help reduce follow-up calls and ensure measurement reliability.
Care team members also have the ability to send personalized messages, reminders and motivational support to the patient's device.
"We are thrilled BPM Pro 2 is now FDA-cleared, this device marks a significant advancement in remote patient monitoring," Antoine Robiliard, vice president of Withings Health Solutions, said in a statement.
"BPM Pro 2 not only delivers precise, clinically meaningful blood pressure and pulse rate measurements but also empowers care teams with real-time insights and direct patient engagement tools. This milestone, along with the recognition as a CES 2025 Innovation Awards Honoree, underscores our commitment to creating solutions that streamline workflows, enhance patient involvement, and set new standards for the quality and reliability of remote care."
THE LARGER TREND
In September, the company secured FDA clearance for its Sleep Rx Mat, a contactless device designed to diagnose sleep apnea at home. The Sleep Rx Mat provides detailed analysis of sleep, breathing and cardiovascular activity throughout the night.
Last year, Withings launched U-Scan, a biomarker assessment platform that can be placed inside a toilet bowl to monitor an individual's health using urine analysis. U-Scan is a reader with changeable analysis cartridges that analyzes urine for specific biomarkers and sends insights to the Withings Health Mate app daily.
In 2022, Withings unveiled Sleep Analyzer, an under-the-mattress sleep-tracking mat designed to detect sleep apnea.
That same year, Medable partnered with Withings Health Solutions to use the company's connected devices for decentralized clinical trials. The partnership allowed Withings' tools for remote monitoring, including connected scales, blood pressure monitors and sleep-tracking mats, to gather patient data and combine with Medable's clinical trial platform.


