FDA Approval
Ben Wolf, partner in Alston & Bird's Health Care Group, told MobiHealthNews how FDA staffing cuts could slow device approvals and what companies can do to stay ahead.
The company’s device, Primary Relief, is the latest of DyAnsys’ long list of devices to receive FDA clearance.
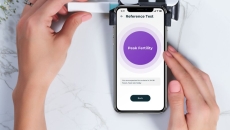
The Inito fertility hormone tracker measures estrogen and luteinising hormone levels and informs users if they ovulate.
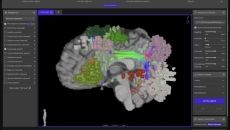
The platform analyses millions of data points from a patient's MRI and delivers insights to neurosurgeons before and during brain surgery.
A collection of 34 approvals and clearances by the US Food and Drug Administration during Q3 and beyond.