Huma
Thyme Care secures $60 million in Series B funding, and Neuspera Medical garners $23 million.
The FDA clearance comes just three months after the company received EU MDR Class IIb certification for its configurable disease management platform.
The U.K.-based company will form an advanced clinical trials division offering digital health solutions for each phase of the development process.
Plus, Northern Ireland’s GPs get prescribing decision support.
Roundup: Atos and Huma enter five-year partnership, Malaffi receives patient data accreditation and…
Also, seven countries begin using EU's travel system.
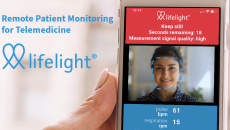
The funds will be used to scale its remote monitoring platform.
The UK study will enable earlier identification of infection and develop research for treatment.
The remote monitoring health tech company’s timely name-change coincides with a radical shift in the way health data is perceived and used, triggered by the coronavirus pandemic.