Medical devices
Butterfly Network, maker of a handheld ultrasound, also announced it had hired Joseph M. DeVivo as the company's new president and CEO.
At HIMSS23, panelists discussed why health systems need to consider the unique needs of their patient populations when implementing remote patient monitoring programs.
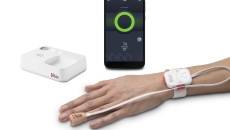
Opioid Halo provides escalating alerts to family and caregivers if it detects signs of opioid-induced respiratory depression.
HIMSS23
Long a security resource, the Medical Device Cybersecurity Regional Incident Preparedness and Response Playbook has gotten some updates. Margie Zuk and Penny Chase of MITRE preview their HIMSS23 session about them.
The company targets to receive a similar clearance from the US FDA by yearend.
HIMSS23
Dr. Shelly Nash, senior vice president and global chief medical information officer at Fresenius Medical Care, discusses the HIMSS23 panel she'll lead on training end-stage kidney disease patients to perform home dialysis using technology.
It has been approved for use in both clinical and home settings.
Also, PowerHealth has developed a new automated interface supporting aged care costing studies.
A study published in The New England Journal of Medicine found a closed-loop control system kept children's glucose levels in the target range longer than standard care.
The wearable device directly measures respiration to predict deterioration in COPD patients.