Public Policy
Bob Watson, CEO of Health Gorilla, explains why providers can expect interoperability enforcement under CMS and the advantages of adoption.
Healthcare experts weigh in on how patients and providers will be impacted due to telehealth and hospital-at-home programs not being extended.
At Healthcare 2030, panelists discussed changes to health plans, cost transparency for consumers and the importance of measuring outcomes.
At Healthcare 2023, panelists highlighted how new primary care and patient communication models are enabling patients to stay more connected to the healthcare system.
At Healthcare 2030, panelists highlighted successful programs at the VA and other government agencies that included integrating digital tools like remote monitoring and AI scribes.
At Healthcare 2030 in Washington, D.C., panelists highlighted debated challenges around interoperability, FHIR adoption and the need for industry-wide standards.
As tariffs target medical devices, plastics and packaging, ArcheHealth CEO Ralph Keiser says technologies that analyze pricing data can help hospitals build evidence for renegotiating contracts and increasing reimbursement.
At an event in Washington, D.C., on Monday, DiMe released a thesis with what it says are four impact areas that are vital to pursue in digital medicine in the next five years.
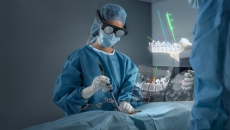
The offering projects real-time 3D visuals of screw placement into a surgeon's field of view to improve accuracy in spine surgery.
Dr. Erik Langhoff, chief medical officer and consultant for the Bronx Regional Health Information Organization, discusses how the agency is addressing safety and efficacy in AI.