Regulation
The Global Digital Health Partnership works to foster international collaboration to strengthen digital health infrastructure, interoperability and system resilience globally.
The AI-enabled technology detects a lack of pulse, confirms the condition using motion sensors and infrared signals, and automatically alerts emergency services if needed.
The firings included 20 individuals in the FDA's Office of Neurological and Physical Medicine Devices.
If finalized, the rule aims to improve the safety of patient data at healthcare institutions and addresses a national security topic by protecting data from bad actors who want to disrupt services, says Eric Avigdor, chief product officer of Votiro.
The EU guidelines highlight prohibitions on harmful manipulation, real-time biometric identification and social scoring.
HIMSS25
Gunnar Trommer of BCG X and Erik Adams of BCG give highlights of their upcoming HIMSS25 panel on the capital needed to develop and commercialize AI-powered medical devices and on completing regulatory milestones like 510(k) clearance.
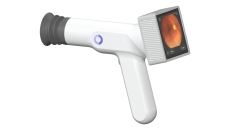
The Sentinel Camera allows point-of-care retinal imaging and eliminates the need for a visit to an eye specialist's office.
Ashkan Afkhami of BCG X sat down with MobiHealthNews during JPM to discuss the U.S. health system's strengths, challenges in cost control and the transformative potential of AI.
President Trump revoked Joe Biden's 2023 order, which required HHS to establish an AI safety program and developers to share test results, among other directives.
At an event outside JPM, which included a representative from HHS, panelists discussed the government's role in advancing AI and the evolving healthcare landscape as a new administration transitions into power.