AI imaging
The company will use the funds to expand the U.S. market and advance its AI-driven technology into new areas.
Researchers claim their invention offers a cost-effective and faster way to do single-cell analysis.
It has also received Australia's regulatory approval for its AI solution.
The companies' joint offering, ThinkSono Guidance, attained Class IIb CE mark approval in March with upcoming plans for FDA approval.
The funds will be used to develop a comprehensive AI imaging model powered by imaging data to improve diagnostic precisions and early detection of conditions.
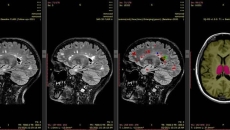
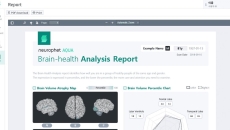
The FDA-cleared and CE-marked image analysis software helps providers diagnose and monitor patients with neurological conditions.
It is making further moves to penetrate the Southeast Asian market.
AI will be integrated to increase pathologists' efficiency.
It seeks to use AI to improve the accuracy of an information-extracting method for structuring radiology reports.
It plans to secure more regulatory approvals for its AI pathology platform.