FDA 510(k)
The company said it's currently focused on managing end-stage kidney disease, but it's investigating using the system for other clinical areas.
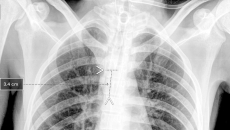
This marks Aidoc's second FDA 510(k) so far this year.
Also: Truepill launches a COVID-19 test coverage platform for insurers, and 23andMe extends its partnership with GSK.
Regulora delivers behavioral therapy based on gut-directed hypnotherapy through a smartphone app.
Providers can use SimpleSense to remotely triage cardio, pulmonary and upper vascular patients.
Also: Nanowear picks up a 510(k) for its remote monitoring undergarments; RxVantage acquires onPoint Oncology.
Alongside the launch of the Rad-G device is the Rad-G sensor, which is intended for both adult and pediatric patients.
The consumer genomics company's existing offering, called AncestryHealth, sold clinician-ordered tests that were not cleared or approved by the FDA.
While the company's new 510(k) cuts down some of the burden, consumers are still advised to consult their healthcare provider before making any changes to their treatments.
The device monitors vitals from up to four feet away, and is designed for use in healthcare settings or at home.