FDA
At The Constellation Forum 2025 in New York, Gottlieb said there are and will continue to be challenges regulating software when it comes to AI devices.
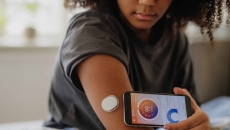
The company's Dexcom Smart Basal will provide patients with a user-friendly option.
Dr. Erik Langhoff, chief medical officer and consultant for the Bronx Regional Health Information Organization, discusses how the agency is addressing safety and efficacy in AI.
Also, a project developing AI for early periodontal disease detection has received over $1 million in grant funding from the Australian government.
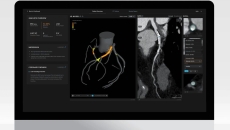
The UroNav system uses MRI and ultrasound images for more precise, minimally invasive care.
The Focalist system aims to combine handheld robotics with real-time imaging to enable clinicians to place needles more precisely.
The Focalist system aims to combine handheld robotics with real-time imaging to enable clinicians to place needles more precisely.
The company will use the funds for a European feasibility study for its transcatheter device and for the launch of a U.S.-based clinical study.
The company will use the funds for a European feasibility study for its transcatheter device and for the launch of a U.S.-based clinical study.
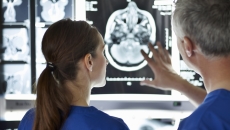
Cirrus uses fMRI imaging to acquire and generate maps of critical brain networks to assist with brain surgery planning.