FDA
The company will use the funding to support the commercial expansion of its product.
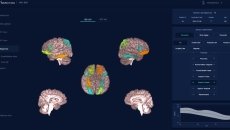
It measures the cortical thickness in a brain scan to provide a patient's level of brain ageing.
It has been approved as a prescription-only software-as-a-medical device in the US.
Also, I-MED will expand its deployment of 4DMedical's lung imaging tool across its network in Australia.
This week's top stories include baby tech company Owlet pulling its connected-sock wearables following an FDA warning letter, and TeamHealth's victory in a lawsuit accusing UnitedHealthcare of engaging in unfair reimbursement practices.
The stock took a tumble in October following the letter.
The news comes about a week after AppliedVR announced it raised $36 million in Series B funding.
Ellume began recalling the tests last month for giving false positive results.
Also, a Māori public health organisation develops a new tool to bring timely COVID-19-related information to Māori people.
The system uses virtual reality and an AI-backed tool.