FDA
Elsa provides a secure platform for scientific reviewers and investigators to access internal documents.
The next-generation Swoop system software enhances image quality for ultra-low-field magnetic resonance imaging.
New NIH office will promote human-based research and scale the use of non-animal approaches to biomedical research.
Lilly accuses four compounders of selling unauthorized versions of the pharma giant's diabetes and weight-loss drugs.
Data from the clinical trial might give patients a new option for treating migraines.
Ben Wolf, partner in Alston & Bird's Health Care Group, told MobiHealthNews how FDA staffing cuts could slow device approvals and what companies can do to stay ahead.
The judge ruled that the FDA overstepped its authority by regulating LDTs as medical devices, overturning the rule and remanding it to the HHS Secretary for review.
The firings included 20 individuals in the FDA's Office of Neurological and Physical Medicine Devices.
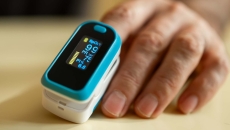
The new draft guidance aims to help improve the accuracy of pulse oximeters in patients across the range of skin pigmentations.
The Hyodol doll, widely marketed as a companion to solo-living seniors, has been registered with the US FDA.