Regulation
The tool uses virtual reality to modify media content in order to help children boost their visual acuity.
The SoftVue system is intended to be used in addition to digital mammograms to screen for cancer among patients with dense breast tissue.
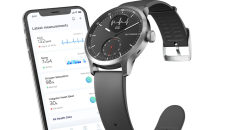
The ScanWatch was first unveiled in January 2020 and is currently on the European market.
The regulator’s report flagged governance issues in the midwifery and pediatrics service.
Healthcare lawyer Heather Alleva lays out the many ways that where you live affects what you can do when it comes to offering virtual care.
The system uses virtual reality and an AI-backed tool.
The FDA said negative test results don’t appear to be affected.
Virginia's ivWatch has just received a three-year Medical Device Marketing Authorization (MDMA) by the Kingdom's Food and Drug Authority.
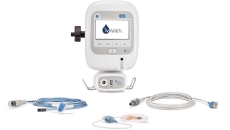
Providers can use SimpleSense to remotely triage cardio, pulmonary and upper vascular patients.
This comes just a month after news broke Nanox was set to acquire Zebra Medical Vision.