Regulation
The Express Scripts app will now allow users to provide proof of COVID-19 vaccination if they received their shot at a pharmacy.
The company’s stock took a nosedive yesterday after it filed an SEC document.
The new clearance expands its insulin dosing support to bolus and premixed insulin titration for Type 2 diabetes.
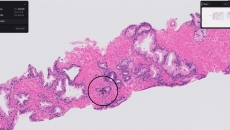
Paige Prostate analyzes digitized biopsy slides and identifies areas that could be cancerous for the pathologist to review further.
Telehealth made up 4.5% of medical claim lines in June, falling from 5% in May.
The company develops AI tools to analyse chest x-rays and breast mammography.
All students over the age of 12 and school staff members must be fully vaccinated in order to be allowed to attend school, the Kingdom’s Ministry of Education said.
The company is also subject to a class action litigation and personal injury claims.
The small study found nearly all patients had a complete abortion without the need for intervention.
HIMSS21
HIMSS Senior VP of Government Relations Tom Leary provides updates on Patient ID Now, pandemic response and the telehealth cliff at HIMSS21.