Regulation
With this designation, Swing is launching a real-world clinical trial to assess its fibromyalgia DTx.
HIMSS21
“I think those APIs for consumers will be as transformative as APIs have been for music, in printing, in travel, in banking and retail,” said Dr. Don Rucker, former ONC National Coordinator for Health IT, at a HIMSS21 lightning session.
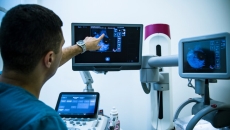
Abbott has received clearance from the FDA for its imaging software that uses artificial intelligence to provide doctors a better view of blood flow and blockages in heart vessels.
This marks the fourth clearance for Volpara’s breast health platform since its original authorization back in 2010.
The system is designated to be used by clinicians to monitor patients at home or in the healthcare setting.
Luxsonic's virtual reality suite, the SieVRt, landed clearance from Health Canada in order to be used in diagnostic radiology.
The move follows recent White House criticism of social media sites for spreading public health misinformation.
Clinical communication tool used in the NHS saved patient photos on smartphones.
The company says it can do all of this without harmfully interfering with other devices.
Masimo is seeking to take Apple's blood oxygenation tech off the U.S. market.